New Regulations of Chinese Customs on Export of Masks
By For Safer Imports • 07/19/2021 • No Comments
In May 2020, for China mask export, the Chinese Customs has upgraded and strictly checked the regulations for civil masks and medical masks. The product packaging should pay attention to below eight points!
(1) We all know,for China mask export, FDA cannot be printed on “all packages and products” of non-medical (civilian), but from April 28 onwards, CE cannot even be allowed except the product is from the “civilian” enterprises that in the “Chamber of Commerce White List” announced on the 12th! (Technical standards such as EN149 cannot be reflected either. In short, foreign standards that have not been certified by the Chamber of Commerce are not allowed to be printed. If an enterprise wants to print, it should contact the Chamber of Commerce to submit a certificate and put it on the white list.)




(2) For medical China mask export, it was a bolt from the blue! In the past, there was a saying about”medical”: Even if a enterprise is with white lists in Announcement No.5 and medical device registration certificates, but it was not included in Announcement 12 “White List of Chambers of Commerce”, still can not use CE mark and FDA mark . At present, the national customs has not unified the regulations on China mask export, some customs will release, some will not release, some will not release today but will release tomorrow. (Shanghai Customs has now made it clear that medical masks that are not on the “Chamber of Commerce White List” will not be released even if the enterprise has a certificate as long as the product&packing are marked by CE and FDA.)

No matter whether it is a medical mask or a medical protective clothing, all medical epidemic prevention products can only be exported according to Chinese standards as long as they are not listed in the white list of the chamber of commerce announced on the 12th. They cannot be declared to conform to foreign standards. Naturally, the packaging should follow the requirements of Chinese standards, and foreign standards such as CE, FDA,EUA and NIOSH cannot be printed at will. Almost 100% of medical manufacturers now have CE or FDA printed on their packaging to some extent, The ones who are shipping can only depend on their luck that they won’t be detected.

The Customs will detain the goods and transfer them to the Customs Anti-smuggling Department.As shown in the figure, more goods are detained than released.
(3) The packaging and certificate of “retail”standard color boxes or color bags must be marked with “Non-medical non-medical” (it can be marked in various languages. In short, it must be marked. Non-standard color boxes or color bags are neutral products. The customs will detain the goods for handling. In addition, the labels must be printed and the paper labels are invalid.)

(4) The packaging of “retail” standard color boxes or color bags must have product implementation standards.Common implementation standards for civil masks are GB2626-2006 or GB/T32610-2016 or other standards.
Attention to Details (1): GB 2626-2006 is generally used for KN95 masks. If this standard is printed on disposable face masks, manufacturers who do not understand basic knowledge will be drawn. Can the customs pass it off?
Attention to Details (2): GB/T 32610-2016 or other higher standards may require individual products in the interior to be packaged in independent “sealed bags” during customs inspection (At present, China mask export requirements are not unified in all regions, depending on the decision of the inspection personnel at that time)


(5) The color box or color bag should not only be printed with the implementation standard, but also the manufacturer, address and other information. The more “regular” info the more “complete” details the better. If it is not printed, it is likely to be judged as neutral packaging or fake and inferior products. Some enterprises of China mask export have doubts: I am a contract manufacturer for foreign customers, and the brand and other information are all from foreign customers. How should I print them? It is very simple, “printing according to the facts” (as shown in the figure): brand holders or traders or sellers can be printed together with the manufacturer’s information. (If it is the contract manufacturing of medical masks, the registration number of medical devices and other license numbers need to be reflected. This is the only product that can prove that this product is in the white list of Announcement No.5. Otherwise, it can only depend on whether China mask export is allowed by the customs.)

(6) The packaging of civil masks should not be printed with words such as filtration, Bacteria/ Virus, or similar signs such as “cross”, and KN95 should not be promoted as N95 or other types. These are all taboos of civil China mask export.


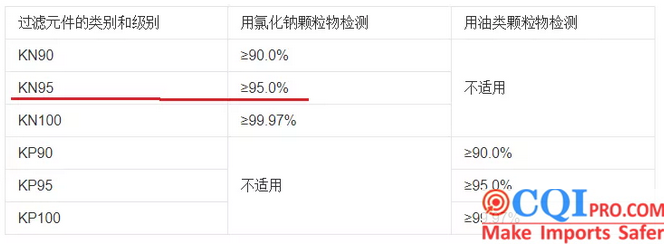
(7)KN95 masks and other flat masks cannot be printed such info as filtering efficiency of N95 mask or reaches: 99% or BFE / VFE>xx%.
The particulate matter filtering effect mentioned in China’s implementation standard GB2626-2006 is only greater than or equal to 95%, and it is not stated that it is purely greater than 95%.
VFE is short for (Virus Filtration Efficiency)
BFE is short for (Bacterial Filtration Efficiency)
PFE is short for (particle filter effect)
If it is a civilian mask, only the filtering effect of PFE can be printed, while VFE and BFE can only be reflected by medical professional masks.
(8) The certificate for China mask export (as shown in the figure) must reflect: manufacturer, address, implementation standard, ingredient content, production batch number, production date, effective date, etc. (These are required on the certificate and are indispensable). The certificate must have a qualified stamp on it. If there is no stamp, the customs can only interpret it as a product that fails the inspection, so the goods are seized.
Pay attention to the composition label of the mask, which must be marked according to the percentage.(Some people will say: My product is medical mask in the white list of China mask export. Do I have to show it like this? We suggest to operate according to the standard, unless you are a confident enterprise in the chamber of commerce white list announced on the 12th. Or at the time of inspection, see the customs mood.)

(The illustration is only a reference sample)
In the mask inspection specified by the importer, the printing of these packages is one of the inspectors’ key inspection contents, followed by the quality of masks to meet the quality and packaging. Such products are the current primary needs of importers.
In addition, update the information needed for the export of epidemic prevention materials, as follows:
This article is an original article for CQI Inspection, who is committed to providing high-quality product inspection technology and know-how sharing for global importers and retailers to make imports safer.
All rights reserved. The contents of this website provided by CQI Inspection may not be reproduced or used without express permission.
For reprint, please contact with CQI Inspection, thank you.
2543 2607 2659 2667 2676 2682 2683 2685 2687 2692 2693 2699 2700 2705 2708 2714 2715 2716 2743 2744 2746 2748 backpack defect check Children furniture quality control China disposable medical gloves Electric toothbrush cost Electric toothbrush inspection Electric toothbrush waterproof inspection Electronic scale quality inspection Glove testing consultation infrared thermometer testing consultation Lighter inspection Lighter process inspection Mask inspection nitrile gloves inspection Pajamas process inspection Plastic clip quality check Quartz watch inspection Quartz watch process inspection Sofa bed inspection solid flooring inspection stainless steel quality control Umbrella process inspection Wenzhou shoes inspection Zhangzhou watch inspection